Integrated Clinical Evaluation.
Clinivation Conformity integrates clinical evaluation into the entire clinical lifecycle with high‐efficiency capture, analysis, and reporting of product performance and safety evidence.
Unlike stand‐alone databases, clinivation Conformity is built for cross‐functional teamwork with personalized, relevant analytics that unlock insights from data to enable decision-making, informed action, and product leadership.

Stand-Alone Clinical Evaluation Impairs Team Progress on Performance and Safety Issues.
The intent of clinical evaluation is to enable informed actions‐ and not simply to inform. These actions may be significant and include redirecting risk management activities, initiating clinical development, revising product labeling, redesigning product interfaces, approving patient access to beneficial therapy, or repositioning the product in the marketplace.
However, initiating significant action usually requires more detailed information than what’s in a packaged, stand‐alone clinical evaluation report. Detailed information, for example, about an issue’s absolute, relative, and comparative magnitude and scope of products affected is usually needed prior to taking action. And each functional area impacted may have their own information requirements, especially in situations where corrective actions or commercial programs must be carefully planned.
Because significant action can’t be initiated without detailed information, stand‐alone clinical evaluation impairs team progress on performance and safety issues.
Connected Clinical Portfolio Strategy Aligns Teams to Deliver the Results That Matter Most.
Despite the most compelling near-term operational objectives, executive leadership must also deliver on all of the organization’s strategic objectives.
This means that teams must always be aligned with resources, milestones, and budgets to deliver the results that matter most.
Integrated Clinical Evaluation with Personalized Analytics Enables Product Leadership.
Clinivation Conformity integrates clinical evaluation across the entire clinical lifecycle, across the target product portfolio, and into the workflows of cross‐functional teams.
Unlike stand‐alone databases, clinivation Conformity is built for cross‐functional teamwork with personalized, relevant analytics that unlock insights from data to enable decision‐making, informed action, and product leadership.
BUILD DATA OVER THE LIFECYCLE.
Capture and refine relevant product data from initial product definition through postmarket outcomes and clinical experience.
VIEW CASE STUDY >
REPURPOSE DATA ACROSS PRODUCTS.
Configure product, indication, cohort, material, and other relationships to apply existing data across the target product portfolio.
CONNECT DATA WITH TEAMS.
Create clinical evaluations for specific functional requirements, including Clinical Evaluation Reports, conformity assessments, and study justifications.
INTEGRATE CLINICAL EVALUATION INTO CROSS-FUNCTIONAL TEAM WORKFLOW
Clinivation Conformity integrates clinical evaluation across the entire clinical lifecycle, across the target product portfolio, and into the workflows of cross‐functional teams.
Unlike stand‐alone databases, clinivation Conformity is built for cross‐functional teamwork with personalized, relevant analytics that unlock insights from data to enable decision‐making, informed action, and product leadership.
Personalized, relevant analytics makes it easy to create clinical evaluations for specific functional requirements, including Clinical Evaluation Reports, conformity assessments, and study justifications.
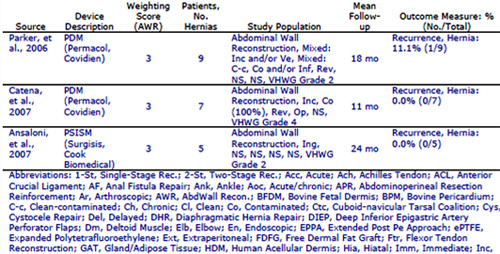
The clinivation Conformity data model enables highly‐specific data analysis to deliver personalized, product‐specific and comparative evidence from across the product portfolio to cross‐functional teams.
- Build Data Across the Entire Product Lifecycle with evidence from publications, internal reports, complaints, market experience, patient registries, vigilance systems, recalls, notifications, hazard alerts, adverse event databases, compassionate use programs, competitor product claims, and more.
- Repurpose Relevant Data Across the Target Product Portfolio by configuring relationships to similar products, indications, applications, intended uses, patient cohorts, device materials, technical characteristics, and more.
- Create Clinical Evaluations for Specific Functional Requirements including conformity assessments and certifications (per Medical Devices Directive), clinical evaluation reports (per MEDDEV 2.7.1), clinical trial design justifications (per ISO 14155), risk management (per ISO 14971), biological safety justifications (per ISO 10993), comparative effectiveness reviews (per AHRQ), commercial product analyses, and more.
- Integrate Product Clinical Performance and Safety With Cross‐Functional Teams including clinical, regulatory, product development, quality, risk management, safety, health economics and outcomes research, and commercial stakeholders.
- Simplify Clinical Development with Intuitive Comparative Evidence Analytics that can slice and group highlygranular product and study data to identify and benchmark relevant prior investigations, study designs, study controls, cohorts, endpoints, confounders, products, indications, applications, investigators, study sites, sponsors, regions, institutions, and more.
- Click‐Through to Content for Real Time Informed Decision Support and immediate access to study documentation, publication libraries, product labeling, risk analyses, and more.
- Runs in clinivation Cloud® to Bring People and Knowledge to the Point of Action and seamlessly interoperates with Clinical Portfolio Strategy, Global Product Value Management, and other clinivation applications.
- Software That’s Ready for Your Teams, Your Infrastructure, and Your Business Processes and seamlessly adapts to your needs‐ leveraging cloud, hybrid, and on‐premises architectures.
- The clinivation clinStart℠ Family of Solution Services Immediately Addresses Your Top Priorities and accelerates a priority‐driven implementation of the clinivation Conformity Platform with a proven approach to this specialized area.
- Clinivation Solution Services Deliver Full Support and include planning, architecture, configuration, development, data migration, validation, documentation, training, launch, support, and program management.
Resources
LIVE PRESENTATION
clinivation Conformity®
SERVICES
clinivation clinStart®
SERVICES
clinivation clinDataXPress®
SERVICES
clinivation Private Technical Training
SERVICES
clinivation Solution Services