Global Market Clearance Management.
The clinivation Global Platform for connected Global Market Clearance Management connects cross-functional teams to address the challenges of execution, effective governance, and lean business performance in their global market clearance operations.
Clinivation Global liberates revenue that’s trapped-in-process by enabling teams to address stall-outs and bottlenecks with transparency, granular reporting, and performance metrics.

MedTech RA- Facing Challenges of Execution, Growth, and Effective Governance.
The recent proliferation of new market clearance regulations and processes in the fast-growing international markets presents new challenges of execution: to effectively deliver larger, more complex market clearance portfolios faster than ever before.
In addition, ongoing industry consolidation means that global market clearance operations must now be capable of not only rapidly integrating new product lines and organizations- but also leveraging infrastructure for business growth and lean business performance.
And contemporary corporate governance now requires executives to implement effective market clearance operational controls to mitigate exposure from myriad compliance risks, penalties, fines, and legal costs.
Connected Global Market Clearance Management- Enabling Scalable Execution and Business Growth.
To address the challenges of execution, clinivation Global enables cross-functional teams to work in scalable global market clearance operations that positions their organization to effectively address an expanded international scope.
Teams have full visibility on their consolidated global market clearance operations- and key performance metrics to find and fix the stall-outs and bottlenecks that trap revenue in inefficient process.
And teams can operate in new markets with access to knowledge and content that is far beyond that which is organic to any department, division, or business unit.
Intuitive dashboards, content unification technology, and streamlined reporting reclaim the team bandwidth that’s now consumed by functioning as a regulatory “help desk,” repeatedly reassembling product dossiers, blindly searching for documentation, and manually preparing routine status reports for senior management.
Enterprise-class systems reclaim the productivity that’s now consumed by the uncertainties of legacy “tracking tools,” home-grown databases, and spreadsheet farms. And integrated architecture accelerates workflow by enabling cross-functional teams to work in synchrony on parallel threads of activity in a manner that properly focuses, builds, and unifies their workflow into tangible, robust market clearance milestones.
Full Transparency of Regulated Operations Enables Contemporary Corporate Governance.
To satisfy the governance requirements on global market clearance operations, clinivation Global provides executives with full transparency on the process and status of their entire global market clearance operations and delivers comprehensive regulatory intelligence into day-to-day activities.
Automated, Clearance-Driven Business Controls Mitigate Global Compliance Risks.
Clinivation Global enables executives to integrate automated, clearance-driven business controls into selling, production, and shipping processes, and thereby mitigate exposure to myriad compliance risks, penalties, fines, and legal costs.
LIBERATE TRAPPED REVENUE.
Liberate revenue that’s trapped-in-process by addressing stall-outs and bottlenecks with transparency, granular reporting, and performance metrics.
![]()
VIEW CASE STUDY >
ENABLE GOVERNANCE AND TRANSPARENCY.
Connect with your teams for full transparency on the process, status, forecast, and compliance of your entire global market clearance operations.
VIEW CASE STUDY >
MITIGATE GLOBAL COMPLIANCE RISKS.
Implement automated, clearance-driven business controls and mitigate exposure to myriad global compliance risks, penalties, fines, and legal costs.
THE PLATFORM FOR CONNECTED GLOBAL MARKET CLEARANCE MANAGEMENT
The clinivation Global Platform serves as the foundation for connected global market clearance management by unifying global market clearance operations into a single management system.
With a complete suite of solution components and technologies, clinivation Global connects cross-functional teams to address the challenges of execution, effective governance, and lean business performance in their global market clearance operations.
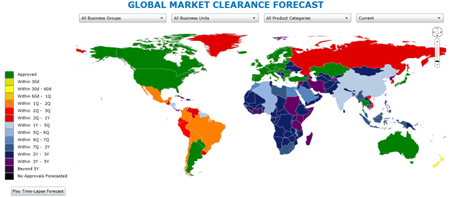
Find and fix bottlenecks and stall-outs with clinivation GlobalSite® Executive Dashboards- with drill-down from enterprise-level to product-level, and click-through to relevant details and source documentation.
- Intuitive, Highly-Configurable Dashboards Deliver Full Transparency on the process, status, forecast, and compliance of your entire global market clearance operations, all easily navigable by region, country, company division, product family, and product.
- Integrated Architecture Enables Cross-Functional Teams to Work in Synchrony on Parallel Threads of Activity in a manner that properly focuses, builds, and unifies their workflow into tangible, robust market clearance milestones.
- Granular Tracking and Reporting Enables Immediate Action on Bottlenecks, Stall-Outs, and Content Gaps with integration of status, forecasts, and performance metrics, granular reporting at each point in the documentation transfer, agency review, and registration processes, and full search functionality with drill-down into the specifics of agency inquiries, submission content, and supporting documentation.
- Content Repurposing into STED Templates Enables High-Speed Submission Cloning for renewals, refilings, and new submissions of the same or additional products into the same or additional countries.
- Simple, Contextual Navigation Delivers Immediate Access to Complete Market Clearance Records including all manufacturing, biocompatibility, sterilization, clinical, compliance, export certification, and agency correspondence documentation- without the need to move or copy documents, or to create or reconfigure file directories or indexes.
- Multidimensional Data Model Represents the Actual Workflow Throughout the Entire Market Clearance Lifecycle in ways that two-dimensional spreadsheet farms can’t possibly function, to simultaneously represent, for example: multiply-amended, multiproduct PMA submission and approval relationships; multiply-refiled, multiproduct, multijurisdictional submission, approval, and renewal relationships; complex kit, accessory, and companion product relationships; and all relevant product registration numbers, dates, and more, for all submissions, renewals, refilings, new submissions, approvals, and expirations.
- Exportable Analytics Simplify Issue Analysis and Presentation and leverage search, user-defined queries, libraries, and templates, for pivot-table functionality and exportability to all desktop applications.
- Built for Integration to Enable Automated Business Controls and Interoperation with selling, production, shipping, ERP, content management, and CRM systems.
- Robust Data Quality, Security, and Administration with audit trail reporting, electronic signatures, multi-level security options, and secure architecture meets or exceeds the requirements of contemporary IT governance policies and 21CFR Part 11.
- Software That’s Ready for Your Infrastructure and Your Business Processes and seamlessly adapts to your needs- leveraging cloud, hybrid, and on-premises architectures.
- The clinivation clearStart Family of Solution Services Immediately Addresses the Top Priorities of Your Global Market Clearance Operations and accelerates a priority-driven implementation of the clinivation Global Market Clearance Management Platform with a proven approach to this specialized area.
- Clinivation Solution Services Deliver Full Support and include planning, architecture, design, configuration, development, data migration, validation, documentation, training, launch, support, and program management.
Resources
LIVE PRESENTATION
clinivation Global®
DATA SHEET
clinivation Global®
DATA SHEET
clinivation GlobalSite®
DATA SHEET
clinivation clearPortERP®
SERVICES
clinivation clearStart®
SERVICES
clinivation clearDataXPress®
SERVICES
clinivation Private Technical Training
SERVICES
clinivation Solution Services